This experiment is for advanced students. Did you know that eating a single peanut will power your brain for 30 minutes? The energy in a peanut also produces a large amount of energy when burned in a flame, which can be used to boil water and measure energy.
Peanuts are part of the bean family, and actually grows underground (not from trees like almonds or walnuts). In addition to your lunchtime sandwich, peanuts are also used in woman’s cosmetics, certain plastics, paint dyes, and also when making nitroglycerin.
What makes up a peanut? Inside you’ll find a lot of fats (most of them unsaturated) and antioxidants (as much as found in berries). And more than half of all the peanuts Americans eat are produced in Alabama. We’re going to learn how to release the energy inside a peanut and how to measure it.
[am4show have=’p9;p50;p88;p91;’ guest_error=’Guest error message’ user_error=’User error message’ ]
Materials:
- raw peanuts
- chemistry stand with glass test tube and holder (watch video)
- flameproof surface (large ceramic tile or cookie sheet)
- paper clip
- alcohol burner or candle with adult help
- fire extinguisher
Download Student Worksheet & Exercises
What’s Going On? There’s chemical energy stored inside a peanut, which gets transformed into heat energy when you ignite it. This heat flows to raise the water temperature, which you can measure with a thermometer. You should find that your peanut contains 1500-2100 calories of energy! Now don’t panic… this isn’t the same as the number of calories you’re allowed to eat in a day. The average person aims to eat around 2,000 Calories (with a capital “C”). 1 Calorie = 1,000 calories. So each peanut contains 1.5-2.1 Calories of energy (the kind you eat in a day). Do you see the difference?
But wait… did all the energy from the peanut go straight to the water, or did it leak somewhere else, too? The heat actually warmed up the nearby air, too, but we weren’t able to measure that. If you were a food scientist, you’d use a nifty little device known as a bomb calorimeter to measure calorie content. It’s basically a well-insulated, well-sealed device that catches nearly all the energy and flows it to the water, so you get a much more accurate temperature reading. (Using a bomb calorimeter, you’d get 6.1-6.8 Calories of energy from one peanut!)
How do you calculate the calories from a peanut?
Let’s take an example measurement. Suppose you measured a temperature increase from 20 °C to 100 °C for 10 grams of water, and boiled off 2 grams. We need to break this problem down into two parts – the first part deals with the temperature increase, and the second deals with the water escaping as vapor.
The first basic heat equation is this:
Q = m c T
Q is the heat flow (in calories)
m is the mass of the water (in grams)
c is the specific heat of water (which is 1 degree per calorie per gram)
and T is the temperature change (in degrees)
So our equation becomes: Q = 10 * 1 * 80 = 800 calories.
If you measured that we boiled off 2 grams of water, your equation would look like this for heat energy:
Q = L m
L is the latent heat of vaporization of water (L= 540 calories per gram)
m is the mass of the water (in grams)
So our equation becomes: Q = 540 * 2 = 1080 calories.
The total energy needed is the sum of these two:
Q = 800 calories + 1080 calories = 1880 calories.
A peanut is not a nut, but actually a seed. In addition to containing protein, a peanut is rich in fats and carbohydrates. Fats and carbohydrates are the major sources of energy for plants and animals.
The energy contained in the peanut actually came from the sun. Green plants absorb solar energy and use it in photosynthesis. During photosynthesis, carbon dioxide and water are combined to make glucose. Glucose is a simple sugar that is a type of carbohydrate. Oxygen gas is also made during photosynthesis.
The glucose made during photosynthesis is used by plants to make other important chemical substances needed for living and growing. Some of the chemical substances made from glucose include fats, carbohydrates (such as various sugars, starch, and cellulose), and proteins.
Photosynthesis is the way in which green plants make their food, and ultimately, all the food available on earth. All animals and nongreen plants (such as fungi and bacteria) depend on the stored energy of green plants to live. Photosynthesis is the most important way animals obtain energy from the sun.
Oil squeezed from nuts and seeds is a potential source of fuel. In some parts of the world, oil squeezed from seeds-particularly sunflower seeds-is burned as a motor fuel in some farm equipment. In the United States, some people have modified diesel cars and trucks to run on vegetable oils.
Fuels from vegetable oils are particularly attractive because, unlike fossil fuels, these fuels are renewable. They come from plants that can be grown in a reasonable amount of time.
[am4show have=’p8;p9;p22;p49;p70;p75;p84;’ guest_error=’Guest error message’ user_error=’User error message’ ]
Materials
- Shelled peanut
- Small pair of pliers
- Match or lighter
- Sink
Download Student Worksheet & Exercises
Procedure
ASK AN ADULT TO HELP YOU WITH THIS EXPERIMENT. DO NOT DO THIS EXPERIMENT BY YOURSELF. The fuel from the peanut can flare up and burn for a longer time than expected.
Close the drain in the kitchen sink. Fill the sink with water until the bottom of the sink is just covered.
Using a small pair of pliers, hold the peanut over the sink containing water. Ask an adult to hold the flame of a lit match or lighter directly under the peanut. When the peanut starts to burn, the lit match or lighter can be removed.
Allow the peanut to burn for one minute. MAKE SURE AN ADULT REMAINS PRESENT AND MAKE SURE TO HOLD THE PEANUT OVER THE SINK. To extinguish the burning peanut, drop it into the water. After you have extinguished the peanut, allow it to cool and then examine it carefully.
Observations
How long does it take for the peanut to start to burn? Does the peanut burn with a clean flame or a sooty flame? What color is the flame? What color does the peanut turn when it burns? Did the size of the peanut change after it has burned for several minutes?
Discussion
You should find that the peanut ignites and burns after a lit match or lighter is held under it for a few seconds. Although you only let the peanut burn for one minute as a safety measure, the peanut would burn for many minutes.
In this experiment, when the peanut burns, the stored energy in the fats and carbohydrates of the peanut is released as heat and light. When you eat peanuts, the stored energy in the fats and carbohydrates of the peanut is used to fuel your body.
Other Things to Try
Hold one end of a piece of uncooked spaghetti in a pair of pliers. Ask an adult to hold the flame of a lit match or lighter under the other end of the spaghetti. When the spaghetti starts to burn, place it in an aluminum pie pan that is in the sink. Make sure to extinguish the burning spaghetti with water when you are finished with the experiment. How does the burning of the spaghetti compare with the burning of the peanut?
Exercises
- What is the process called where plants get food from the sun?
- Osteoporosis
- Photosynthesis
- Chlorophyll
- Metamorphosis
- Where does all life on the planet get its food?
- List two ways that we could use the energy in a peanut:
[/am4show]
Click here to go to next lesson on Thermostat.

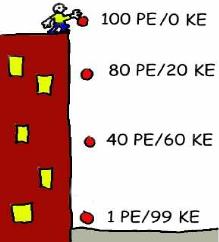 As Phillip holds the ball at the top of the building, the ball has 100 Joules of potential energy (the number is just an example). When he drops it, the potential energy of the ball drops since the height of the ball gets less and less. At the same time, however, kinetic energy increases because the speed of the ball increases. All the way down, the sum of the two energies equals 100. No energy gets lost, it only gets transferred. Energy is conserved.
Now here’s a question you may be asking yourself, “If energy is neither created or destroyed in a closed system then why doesn’t a pendulum swing forever?”
That’s a very good question. Energy is neither created or destroyed, but it can be transferred into non- useful energy.
In the case of a pendulum, every swing loses a little bit of energy,which is why each swing goes slightly less high (achieves slightly less PE) than the swing before it. Where does that energy go? To heat. The second law of thermodynamics states basically that eventually all energy ends up as heat. If you could measure it, you’d find that the string, and the weight have a slightly higher temperature then they did when they started due to friction. The energy of your pendulum is lost to heat!
If you could prevent the loss of energy to useless energy, you could create a perpetual motion machine. A machine that works forever! There have been a lot of folks who have spent a lot of time trying to make a perpetual motion machine. So far, they have all failed. A perpetual motion machine is one that is said to be 100% energy efficient. In other words all the energy that goes into it goes to useful energy.
Your pendulum could be said to be about 90% efficient.Very little energy is converted into useless energy. In most systems, energy is converted to useless heat and sound energy.
As Phillip holds the ball at the top of the building, the ball has 100 Joules of potential energy (the number is just an example). When he drops it, the potential energy of the ball drops since the height of the ball gets less and less. At the same time, however, kinetic energy increases because the speed of the ball increases. All the way down, the sum of the two energies equals 100. No energy gets lost, it only gets transferred. Energy is conserved.
Now here’s a question you may be asking yourself, “If energy is neither created or destroyed in a closed system then why doesn’t a pendulum swing forever?”
That’s a very good question. Energy is neither created or destroyed, but it can be transferred into non- useful energy.
In the case of a pendulum, every swing loses a little bit of energy,which is why each swing goes slightly less high (achieves slightly less PE) than the swing before it. Where does that energy go? To heat. The second law of thermodynamics states basically that eventually all energy ends up as heat. If you could measure it, you’d find that the string, and the weight have a slightly higher temperature then they did when they started due to friction. The energy of your pendulum is lost to heat!
If you could prevent the loss of energy to useless energy, you could create a perpetual motion machine. A machine that works forever! There have been a lot of folks who have spent a lot of time trying to make a perpetual motion machine. So far, they have all failed. A perpetual motion machine is one that is said to be 100% energy efficient. In other words all the energy that goes into it goes to useful energy.
Your pendulum could be said to be about 90% efficient.Very little energy is converted into useless energy. In most systems, energy is converted to useless heat and sound energy.
 If you’re finding that the marbles fall out before the bobsled reaches the bottom of the slide, you need to either crimp the foil more closely around the marbles or decrease your hill height.
If you’re finding that the marbles fall out before the bobsled reaches the bottom of the slide, you need to either crimp the foil more closely around the marbles or decrease your hill height. Imagine an arrow is shot from a bow and by the time it hits an apple it is traveling with 10 Joules of kinetic energy (kinetic energy is the energy of motion). What’s meant by kinetic energy is that when it hits something, it can do that much work on whatever is hit.
Imagine an arrow is shot from a bow and by the time it hits an apple it is traveling with 10 Joules of kinetic energy (kinetic energy is the energy of motion). What’s meant by kinetic energy is that when it hits something, it can do that much work on whatever is hit.