When I was in grad school, I needed to use an optical bench to see invisible things. I was trying to ‘see’ the exhaust from a new kind of F15 engine, because the aircraft acting the way it shouldn’t – when the pilot turned the controls 20o left, the plane only went 10o. My team had traced the problem to an issue with the shock waves, and it was my job to figure out what the trouble was. (Anytime shock waves appear, there’s an energy loss.)
Since shock waves are invisible to the human eye, I had to find a way to make them visible so we could get a better look at what was going on. It was like trying to see the smoke generated by a candle – you know it’s there, but you just can’t see it. I wound up using a special type of photography called Schlieren.
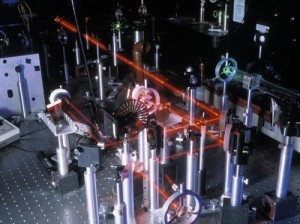 An optical table gives you a solid surface to work on and nails down your parts so they don’t move. This is an image taken with Schlieren photography. This technique picks up the changes in air density (which is a measure of pressure and volume).
An optical table gives you a solid surface to work on and nails down your parts so they don’t move. This is an image taken with Schlieren photography. This technique picks up the changes in air density (which is a measure of pressure and volume).
The air above a candle heats up and expands (increases volume), floating upwards as you see here. The Schlieren technique shines a super-bright xenon arc lamp beam of light through the candle area, bounces it off two parabolic mirrors and passes it through a razor-edge slit and a neutral density filter before reaching the camera lens. With so many parts, I needed space to bolt things down EXACTLY where I wanted them. The razor slit, for example, just couldn’t be anywhere along the beam – it had to be right at the exact point where the beam was focused down to a point.
I’m going to show you how to make a quick and easy optical lab bench to work with your lenses. Scientists use optical benches when they design microscopes, telescopes, and other optical equipment. You’ll need a bright light source like a flashlight or a sunny window, although this bench is so light and portable that you can move it to garage and use a car headlight if you really want to get creative. Once your bench is set up, you can easily switch out filters, lenses, and slits to find the best combination for your optical designs. Technically, our setup is called an optical rail, and the neat thing about it is that it comes with a handy measuring device so you can see where the focal points are for your lenses. Let’s get started:
[am4show have=’p8;p9;p19;p46;p105;p66;p89;’ guest_error=’Guest error message’ user_error=’User error message’ ]
Materials:
- lenses (glass or plastic), magnifying lenses work also
- two razor blades (new)
- index cards (about four)
- razor
- old piece of wood
- single hair from your head
- tape
- aluminum foil
- clothespins (2-4)
- laser pointer
- popsicle sticks (tongue-depressor size)
- hot glue gun
- scissors and a sharp razor
- meter sticks (2)
- bright light source (ideas for this are on the video)
Your lenses are curved pieces of glass or plastic designed to bend (refract) light. A simple lens is just one piece, and a compound lens is like the lens of a camera – there’s lots of them in there. The first lenses were developed by nature – dewdrops on plant leaves are natural lenses. The light changes speed and bends when it hits the surface of the drop, and things under the drop appear larger. (Read more about refraction here.) The earliest written records of lenses are found in the Greek archives and described as being glass globes filled with water.
Concave Lenses
Concave lenses are shaped like a ‘cave’ and curve inward like a spoon. Light that shines through a concave lens bends to a point (converging beam). Ever notice how when you peep through the hole in a door (especially in a hotel), you can see the entire person standing on the doorstep? There’s a concave lens in there making the person appear smaller.
You’ll also find these types of lenses in ‘shoplifting mirrors’. Store owners post these mirrors around help them see a larger area than a flat mirror shows, although the images tend to be a lot smaller.
If you have a pair of near-sighted glasses, chances are that the lenses are concave. Near-sighted folks need help seeing things that are far away, and the concave lenses increase the focal point to the right spot on their retina.
Concave lenses work to make things look smaller, so there not as widely used as convex lenses. You’ll find concave lenses inside camera lenses and binoculars to help clear weird optical problems that happen around the edges of a convex lens (called aberration).
Here’s a video on lenses, both convex and concave:
Convex lenses bulge outwards, bending the light out in a spray (diverging beam). A hand-held magnifying glass is a single concave lens with a handle. These lenses have been used as ‘burning glasses’ for hundreds of years – by placing a small piece of paper at its focal point and using the sun as a light source, you can focus the light energy so intensely that you reach the flash point of the paper (the paper auto-ignites around 450oF).
When you stack a large convex lens above a solar panel, the magnification effect makes it so you can get away with using a smaller photovoltaic cell to get the same amount of energy from the sun. You’ll find convex lenses in telescopes, microscopes, binoculars, eyeglasses, and more.
Mirrors
 What if you coat one side of the lens with a reflecting silver coating? You get a mirror!
What if you coat one side of the lens with a reflecting silver coating? You get a mirror!
Stick wooden skewers into a piece of foam to simulate how the light rays reflect off the surface of the mirror. Note that when the mirror (foam) is straight, the light rays are straight (which is what you see when you look in the bathroom mirror). The light bounces off the straight mirror and zips right back at you, remaining parallel.
 Now arch the foam. Notice how the light ways (skewers) come to a point (focal point).
Now arch the foam. Notice how the light ways (skewers) come to a point (focal point).
After the focal point, the rays invert, so the top skewer is now at the bottom and the bottom is now at the top. This is your flipped (inverted) image. This is what you’d see when you look into a concave mirror, like the inside of a metal spoon. You can see your face, but it’s upside-down.
Slits
A slit allows light from only one source to enter. If you have light from other sources, your light beam is more scattered and your images and lines become blurry. Thin slits can be easily made by placing the edges of two razor blades very close together and securing into place. We’re going to use an anti-slit using a piece of hair, but you can substitute a thin needle.
Here’s a video on using filters and slits with your laser:
Filters
There are hundreds of different types of filters, used in photography, astronomy, and sunglasses. A filter can change the amount and type of light allowed through it. For example, if you put on red-tinted glasses, suddenly everything takes on a reddish hue. The red filter blocks the rest of the incoming wavelengths (colors) and only allows the red colors to get to your eyeball. There are color filters for every wavelength, even IR and UV.
UV filters reduce the haziness in our atmosphere, and are used on most high-end camera lenses, while IR filters are heat-absorbing filters used with hot light sources (like near incandescent bulbs or in overhead projectors).
A neutral density (ND) filter is a grayish-colored filter that reduces the intensity of all colors equally. Photographers use these filters to get motion blur effects with slow shutter speeds, like a softened waterfall.
Build an Optical Bench
It’s time to put all these pieces together and make cool optical stuff – are you ready?
Download Student Worksheet & Exercises
Click here for more experiments on building your own microscope and telescope.
Cat’s Eyes
Corner reflectors are U-turns for light beams. A corner mirror made from three mirrors will reflect the beam straight back where it came from, no matter what angle you hit it at. Astronauts placed these types of mirrors on the moon so scientists could easily bounce laser beams off the moon and have them return to the same place on Earth. They used these reflected laser beams to measure the speed of light.
You’ll find corner mirrors in “cat’s eye” reflectors on the road. Car headlights illuminate the reflectors and send the beam straight back the same way – right at the driver.
Exercises
- Using only the shape, how can you tell the difference between a convex and a concave lens?
- Which type of lens makes objects viewed through it appear smaller?
- Which type of lens makes the objects viewed through it appear larger?
- How do you get the f number?
[/am4show]
Click here to go to next lesson on Convex Lenses



 Everyone old enough to remember the Rubik’s Cube craze of the 1980s in the USA also remembers how it was near impossible to solve the thing! Originally created by a professor of architecture Erno Rubik, it was sold to a toy company in 1980 as the “Magic Cube”.
Everyone old enough to remember the Rubik’s Cube craze of the 1980s in the USA also remembers how it was near impossible to solve the thing! Originally created by a professor of architecture Erno Rubik, it was sold to a toy company in 1980 as the “Magic Cube”.



 When a beam of light hits a different substance (like the water), the wavelength changes because the speed of the light changes. If you’re thinking that the speed of light is always constant, you’re right… in a vacuum like outer space between two reference frames.
When a beam of light hits a different substance (like the water), the wavelength changes because the speed of the light changes. If you’re thinking that the speed of light is always constant, you’re right… in a vacuum like outer space between two reference frames.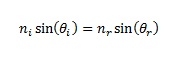
 Spectrometers are used in chemistry and astronomy to measure light. In astronomy, we can find out about distant stars without ever traveling to them, because we can split the incoming light from the stars into their colors (or energies) and “read” what they are made up of (what gases they are burning) and thus determine their what they are made of. In this experiment, you’ll make a simple cardboard spectrometer that will be able to detect all kinds of interesting things!
Spectrometers are used in chemistry and astronomy to measure light. In astronomy, we can find out about distant stars without ever traveling to them, because we can split the incoming light from the stars into their colors (or energies) and “read” what they are made up of (what gases they are burning) and thus determine their what they are made of. In this experiment, you’ll make a simple cardboard spectrometer that will be able to detect all kinds of interesting things! An optical table gives you a solid surface to work on and nails down your parts so they don’t move. This is an image taken with Schlieren photography. This technique picks up the changes in air density (which is a measure of pressure and volume).
An optical table gives you a solid surface to work on and nails down your parts so they don’t move. This is an image taken with Schlieren photography. This technique picks up the changes in air density (which is a measure of pressure and volume). What if you coat one side of the lens with a reflecting silver coating? You get a mirror!
What if you coat one side of the lens with a reflecting silver coating? You get a mirror! Now arch the foam. Notice how the light ways (skewers) come to a point (focal point).
Now arch the foam. Notice how the light ways (skewers) come to a point (focal point).