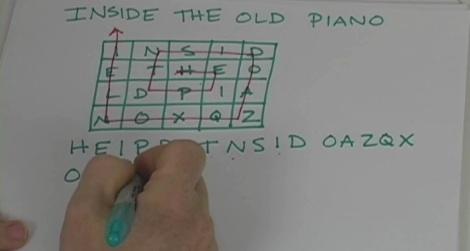
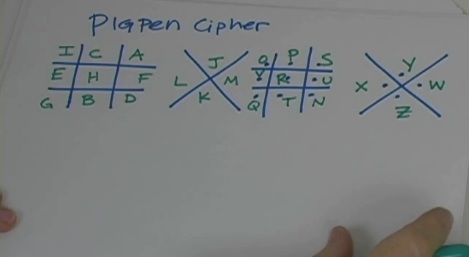
“CQ, CQ.. calling CQ 20 meters. This is KJ6ZFH calling. Kilo Juliet Six Zulu Foxtrot Hotel in San Luis Obispo calling CQ 20 meters. Hello CQ, CQ, CQ 20 meters. This is KJ6ZFH calling. Kilo Juliet Six Zulu Foxtrot Hotel in San Luis Obispo calling CQ 20 meters and standing by for a call.”
“CQ, CQ.. calling CQ 20 meters. This is KJ6ZFH calling. Kilo Juliet Six Zulu Foxtrot Hotel in San Luis Obispo calling CQ 20 meters. Hello CQ, CQ, CQ 20 meters. This is KJ6ZFH calling. Kilo Juliet Six Zulu Foxtrot Hotel in San Luis Obispo calling CQ 20 meters and standing by for a call.”
What is all THAT?
Well, it’s a “ham” radio operator calling out to anyone listening on a special radio frequency (the 20 meter band) inviting them to answer and talk. It’s a fun and exciting hobby for those who love electronics and meeting new people from all over the world. Plus it’s an ideal hobby for students because it’s inexpensive (there’s no on-going cost once you’ve bought your radio, unlike a cell phone plan), provides a huge amount of learning opportunities from geography to electronics to foreign language, and it’s just plain cool.
 Amateur radio has been around for a long time (since 1912), so you never know who you’re going to make contact with when you put out a call. A friend of mine actually talked with the King of Jordan once… no kidding!
Amateur radio has been around for a long time (since 1912), so you never know who you’re going to make contact with when you put out a call. A friend of mine actually talked with the King of Jordan once… no kidding!
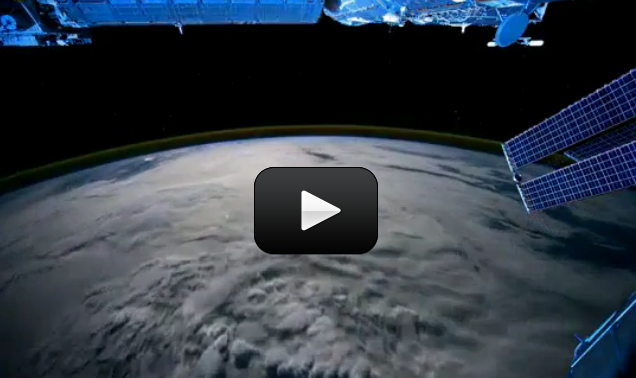
You can even connect with astronaut hams on the International Space Station (see the image on the left… notice her hair?) Suni Williams is using the space station’s radio module to communicate with kids on earth. There’s really no limit to what you can do once you learn the fundamentals.
I’m going to outline the basics of amateur radio so you can really get a feel for what it is, why people still love it, and what it’s really like out there in the air. Let’s start with the first thing people always ask me, which is: “What is CQ?” “CQ” means “calling any station”. Calling “CQ” takes about 20-30 seconds to say clearly, and includes the call sign (KJ6ZFH) several times, including phonetically (Kilo Juliet Six Zulu Foxtrot Hotel). Also includes the location information so the listener knows exactly who and where the caller is.
The videos on this page were created by Tyler, and his call sign is N7TFP. He’s extremely passionate about amateur radio, and he has created several videos on the hobby that I thought you might enjoy, including a peek at his radio shack. The “shack” refers to the place you do your “hamming”, usually in a shed or room where all your radio equipment is located. Some hams operate from their homes, others wire up their vehicles with portable radio stations, some hams set up a separate building in the yard, and a few had to be sneaky about hamming, especially if their housing development or family didn’t approve of their rooftop antennas. In fact, I know of one ham that was so desperate to continue his hobby, despite his wife’s objections that he stashed his radio equipment in a tiny closet and figured out a way to use the metal rain gutters as his antenna!
Let’s take a look at how a ham operator works:
If you’ve got your Amateur Radio License (we’ll talk more on how to do that later), you are ready to get on the air! First thing you should do is listen to how other hams do their communication so you can pick up on a few things. Different bands have different approaches to communication, so it really helps to hear a couple of exchanges before you make your first contact. Most people start with a Technician license with either a handi-talkie (hand-held transceiver) or a portable radio by going through something called a local “repeater”. Let me explain: The most popular way to communicate for a newly licensed ham to talk with other hams is through a local repeater. It’s a two-way radio system that receives communication on one frequency and then re-transmits what it hears on another frequency at exactly the same time. It’s simply a machine that relays your message since your transceiver has a limited range. It’s limited because of the size of the antenna and how the earth curves. A repeater can get your signal to reach a much further listener this way. There are repeaters all over the country. To make a contact on the 2 meter band through a repeater, I press the mike button and say:
-
- “KJ6ZFH listening.” (Make sure you use your own call sign!). Now this may be all I need to do in order to get a response. But more often than not, there’s no response at all. So then I try again but add in a little more information like this:
- “KJ6ZFH is monitoring and listening for a call.” Usually I don’t need to call CQ on a repeater, although there’s nothing in the rule books that say you can’t do that. I’ll tell you more about calling CQ in a moment.
- When you get a response, it might sound something like this:
- “KJ6ZFH this is W2AYL in St. George returning. My name is Pat. Back to you. W2AYL”. Then I’d wait for the repeater’s tone for the go-ahead to proceed with communication.
- I’d press my mike button and respond, because now I’m in! Sometimes I give my name and location or any other info I want to talk to Pat about.
- When I’m done talking, I say: “Over” or “Back to you.” I also make sure to give my call sign frequently (you’re required to do this every 10 minutes minimum). An easy way to do this is to tack it on at the end: “W2AYL this is KJ6ZFH. Over.”
- When I’m done talking with Pat but still want to continue to monitor, I say goodbye by saying 73 (which means ‘best wishes’) and sign off like this: “W2AYL 73, this is KJ6ZFH clear and monitoring.”
- If I’m done for the night, I would say: “W2AYL 73, this is KJ6ZFH clear and QRT” instead.
And that’s it! It’s really that simple. And it exciting and fun to keep track of all the different folks you get to talk with all over the country and world.
Make Contact by Calling CQ
“CQ” means “calling any station”. Some licenses permit you to operate on different bands, like SSB on 10 meters. Here’s what you’d do in this case: You always start by finding a clear frequency. Let’s say you’ve chosen to operate on 28.460. You want to speak clearly and ask “Is this frequency in use? This is KJ6ZFH.” If you don’t get a response, ask a second time just to be sure. If there’s still no response, you can continue. However, note that if the frequency is in use, just move to another frequency and try again.
Now you get to call: “CQ CQ CQ. This is Kilo Juliet Six Zulu Foxtrot Hotel calling CQ CQ CQ. This is Kilo Juliet Six Zulu Foxtrot Hotel, KJ6ZFH calling CQ and waiting for a call.” Wait and listen for a return call. If you’re on an HF (high frequency) band like 10 meters, the signal you get back might be very strong to very weak or anything in between, so use your ears here.
You may hear “Kilo Juliet Six Zulu Foxtrot Hotel this is (their call sign) calling.” You respond by saying “(Their call sign, using phonetics) this is KJ6ZFH. Thanks for the call your signal is 59. My name is Aurora and my QTH is San Luis Obispo. So how do you copy? (Their call sign) this is KJ6ZFH over.”
You made HF (high frequency) contact! You can make the contact as you wish depending on the band conditions and what you find to discuss with your new friend in a new country!
Q Signals
Q signals are used primarily in CW. They provide an abbreviated way of asking a question or making a statement. A Q signal followed by a question mark (?) asks a question. A Q signal without the “?” answers the question or makes the statement.
The following are Q signals commonly used by CW operators world wide:
-
- QSY – Shall I change to to transmission on another frequency? Change transmission to another frequency (or ___ kHz).
- QTH – What is your location? My location is ___.
- QRZ – Who is calling me? You are being called by ___ (on ___ kHz).
- QSL – Can you acknowledge receipt? I am acknowledging receipt.
- QSO – Can you communicate with ___ direct or by relay? I can communicate with ___ direct (or by relay through ___).
- QRP – Shall I decrease power? Decrease power.
- QRM – Is my transmission being interferred with? Your transmission is being interferred with ___. (1. Nil 2. Slightly 3. Moderately 4. Severely 5. Extremely)
- QRN – Are you troubled by static? I am troubled by static —. (1-5 as under QRM)
You can find a complete list of Q signals here.
Building Your Amateur Radio Station
If you’re ready to build your own amateur radio station, here’s a video to get you started:
Your First Radio
This is a video on selecting your first radio. Some folks feel that a HT (handi-talkie, or hand-held transceivers) does not make good first radios, however for kids it’s a great first step into the hobby, and it’s not a huge investment… and it comes with the antenna and everything you need, all in one package. You can get a Baofang UV-5R if you’d like an inexpensive handi-talkie for about $60.
If you’d like a larger radio, get yourself a portable radio 30-60W in the 2 meter / 70cm bands – this will keep you busy for a long time. My first radio was the Yaseu 7900.
Sometimes, you’ll hear reference to the word “oscilloscope” or “o-scope”. If you’re wondering what it is, it an instrument used in the electronics/electrical fields (which includes radio) to help hams keep their equipment working properly. Most folks get their first exposure to one of these in a college level lab, but here’s sneak peek so you don’t have to wait. Here’s how it works:
The first thing you’ll want to do is set up your radio to the appropriate frequency, offset, CTCSS tone (if needed) so you’ll be ready to make your contact – all of this is covered in your instruction manual that comes with your radio.
Silly as this sounds, I spent to much time getting my equipment dialed in right that when it came time to speak, I clean forgot my call sign! I had to stick it (KJ6ZFH) on a post-it note right in front of me so I wouldn’t forget.
Getting Your Amateur Radio License
After watching these videos and learning about radio communication, are you ready to get your license? If you’re excited about the idea, then NOW is a great time to jump on it. Would it surprise you to know that when I got my license, I woke up the morning of the test without even knowing that I was about to take the test? Not only that, I had not even cracked open an book on the subject? Sure, there are only 35 questions to answer, and they take them from a published pool of 350 questions, but honestly, it was a surprise date from my husband.
He drove me to a testing center where we studied for 5 hours, then took the test… all in a single day! No long weeks of studying theory, no late nights or giving up other activities… just a day of studying, take the test, and boom! I got my license. It cost me $20. So I totally encourage you to do it now while you’re excited and have momentum in this direction… that’s the moment you make the decision that takes you in a direction you really want to go. You can look up information on testing at the ARRL.
Enough said – here’s a quick video on getting your license you might find helpful:


























































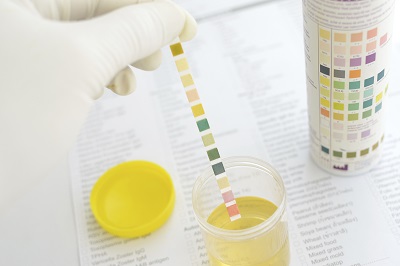
 Although urine is sterile, it has hundreds of different kinds of wastes from the body. All sorts of things affect what is in your urine, including last night’s dinner, how much water you drink, what you do for exercise, and how well your kidneys work in the first place. This experiment will show you how the kidneys work to keep your body in top shape.
Although urine is sterile, it has hundreds of different kinds of wastes from the body. All sorts of things affect what is in your urine, including last night’s dinner, how much water you drink, what you do for exercise, and how well your kidneys work in the first place. This experiment will show you how the kidneys work to keep your body in top shape.





 “CQ, CQ.. calling CQ 20 meters. This is KJ6ZFH calling. Kilo Juliet Six Zulu Foxtrot Hotel in San Luis Obispo calling CQ 20 meters. Hello CQ, CQ, CQ 20 meters. This is KJ6ZFH calling. Kilo Juliet Six Zulu Foxtrot Hotel in San Luis Obispo calling CQ 20 meters and standing by for a call.”
“CQ, CQ.. calling CQ 20 meters. This is KJ6ZFH calling. Kilo Juliet Six Zulu Foxtrot Hotel in San Luis Obispo calling CQ 20 meters. Hello CQ, CQ, CQ 20 meters. This is KJ6ZFH calling. Kilo Juliet Six Zulu Foxtrot Hotel in San Luis Obispo calling CQ 20 meters and standing by for a call.” Amateur radio has been around for a long time (since 1912), so you never know who you’re going to make contact with when you put out a call. A friend of mine actually talked with the King of Jordan once… no kidding!
Amateur radio has been around for a long time (since 1912), so you never know who you’re going to make contact with when you put out a call. A friend of mine actually talked with the King of Jordan once… no kidding!