Since we can’t see soundwaves as they move through the air, we’re going to simulate one with rope and a friend. This will let you see how a vibration can create a wave. You’ll need at least 10 feet of rope (if you have 25 or 50 feet it’s more fun), a piece of tape (colored if you have it), a slinky, and a partner. Are you ready?
[am4show have=’p8;p9;p11;p38;p92;p16;p43;p64;p75;’ guest_error=’Guest error message’ user_error=’User error message’ ]
1. Give one end of the rope to your partner.
2. Stretch the rope out so that it is a bit slack.
3. Now move your hand up and down. Feel free to do it several times in a row. Your partner should keep his or her hands as still as possible.
4. Watch the waves move from your hand to the other end of the rope.
5. Now let your partner create waves.
6. If you wish, you can try to time your vibrations and create waves with specific frequencies. A frequency of one Hertz is fairly easy to do (one rope shake per second). Can you create rope waves of higher frequencies? You may find that your arm gets tired pretty quickly!
Your hand is the vibrating particle. As your hand vibrated up and down, you moved the particles of the rope up and down. As those particles of rope vibrated, they vibrated the particles next to them. As they vibrated, they vibrated the particles next to them and so on and so forth. So the wave moved from your hand across the room. Did your hands move across the room. Nope, but the wave you created with your vibrating hand did.
This is the way energy travels. Why is the rope wave energy? Because the particles moved a distance against a force. Work was done on the particles. In fact, when you shook the rope, your energy from your body moved across the room with the wave and was transferred (moved to) your partner. Your partner’s hands could feel the energy you put into the rope in the first place. The work you did on the rope was transferred by the rope wave and did work on your partners hand. You have moved energy across the room!
Now… let’s add another element to this experiment…
Transverse Waves
1. Put a piece of (colored if possible) tape in about the middle of the rope.
2. Tie your rope to something or let your friend hold on to one end of it.
3. Now pull the rope so that it is a bit slack but not quite touching the floor.
4. Vibrate your arm. Move your arm up and down once and watch what happens.
5. Now, vibrate your arm a bunch of times (not too fast) and see the results. Notice the action of the tape in the middle of the rope.
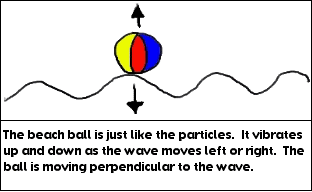 What you’ve done is create a transverse wave. With a transverse wave, if the particle (in this case your hand) moves up and down, the wave will move to the left and/or right of the particle. The word perpendicular means that if one thing is up and down, the other thing is left and right. A transverse wave is a wave where the particle moves perpendicular to the medium. The medium is the material that’s in the wave. The medium in this case is the rope.
What you’ve done is create a transverse wave. With a transverse wave, if the particle (in this case your hand) moves up and down, the wave will move to the left and/or right of the particle. The word perpendicular means that if one thing is up and down, the other thing is left and right. A transverse wave is a wave where the particle moves perpendicular to the medium. The medium is the material that’s in the wave. The medium in this case is the rope.
For example, in a water wave, the medium is the water. Your hand moved up and down, but the wave created by your hand moved across the room, not up. The wave moved perpendicular to the motion of your hand. Did you take a look at the tape? The tape represents a particle in the wave. Notice that it too, was going up and down. It was not moving along the wave. In any wave the particles vibrate, they do not move along the wave.
Longitudinal Waves
Now that you’ve seen a transverse wave, let’s take a look at a longitudinal wave. Here’s what you do:
1. Put a piece of tape on one slinky wire in the middle or so of the slinky.
2. Let your friend hold on to one end of the slinky or anchor the slinky to a chair or table.
3. Now stretch the slinky out, but not too far.
4. Quickly push the slinky toward your friend, or the table, and then pull it back to its original position. Did you see the wave?
5. Now do it again, back and forth several times and watch where the slinky is bunched up and where it’s spread out.
6. Notice the tape. What is it doing?
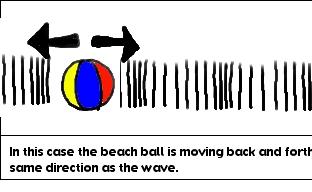 Here you made a longitudinal wave. A longitudinal wave is where the particle moves parallel to the medium. In other words, your hand vibrated in the same direction (parallel to the direction) the wave was moving in. Your vibrating hand created a wave that was moving in the same direction as the hand was moving in. Did you take a look at the tape? The tape was moving back and forth in the same direction the wave was going.
Here you made a longitudinal wave. A longitudinal wave is where the particle moves parallel to the medium. In other words, your hand vibrated in the same direction (parallel to the direction) the wave was moving in. Your vibrating hand created a wave that was moving in the same direction as the hand was moving in. Did you take a look at the tape? The tape was moving back and forth in the same direction the wave was going.
Do you see the difference between a transverse wave and a longitudinal wave? In a transverse waves the particles vibrate in a different direction (perpendicular) to the wave. In a longitudinal wave the particles vibrate in the same direction (parallel) to the wave.
What’s the Difference between Amplitude and Wavelength?
Here’s an easy way to get a feel for amplitude:
1. Put a piece of tape in about the middle of the rope.
2. Tie your rope to something or let your friend hold on to one end of it.
3. Now pull the rope so that it is a bit slack but not quite touching the floor.
4. Your friend should hold their hands as still as possible.
5. Vibrate your hand but only move it up and down about a foot or so. Have your partner pay attention to how that feels when the wave hits him or her.
6. Now, vibrate your hand but now move it up and down 2 or 3 feet. How does that feel to your partner?
7. Have your partner do the vibrating now and see what you feel.
You created two different amplitude waves. The first wave had a smaller amplitude than the second wave. What you and your partner should have felt was more energy the second time. The wave should have hit your hand with more energy when the wave had more amplitude.
Here’s a great way to visualize wavelength:
1. Tie your rope to something or let your friend hold on to it.
2. Now pull the rope so that it is a bit slack but not quite touching the floor.
3. Your friend should hold their hands as still as possible.
4. Now begin vibrating your hand fairly slowly. In this case, it works better if you move your hand in a circle.
5. Try to make a wavelength with the rope. In other words it will look like you’re playing jump rope.
6. Now try a one and a half wavelengths.
7. Can you get two or more wavelengths? You’ve really got to get your hand moving to get it.

In this image, the left wave is ONE wavelength, the middle is 1.5 wavelegnths, and the right is TWO wavelengths. See the difference?
Did you notice how the frequency of your hand determined the wavelength of the rope? The faster your hand, moved the more wavelengths you could get.
[/am4show]







 Flowering plants can be divided into monocotyledons and dicotyledons (monocots and dicots). The name is based on how many leaves sprout from the seed, but there are other ways to tell them apart. For monocots, these will be in multiples of three (wheat is an example of a monocot). If you count the number of petals on the flower, it would have either three, six, nine, or a multiple of three. For dicots, the parts will be in multiples of four or five, so a dicot flower might have four petals, five petals, eight, ten, etc.
Flowering plants can be divided into monocotyledons and dicotyledons (monocots and dicots). The name is based on how many leaves sprout from the seed, but there are other ways to tell them apart. For monocots, these will be in multiples of three (wheat is an example of a monocot). If you count the number of petals on the flower, it would have either three, six, nine, or a multiple of three. For dicots, the parts will be in multiples of four or five, so a dicot flower might have four petals, five petals, eight, ten, etc.

 When birds and animals drink from lakes, rivers, and ponds, how pure it is? Are they really getting the water they need, or are they getting something else with the water?
When birds and animals drink from lakes, rivers, and ponds, how pure it is? Are they really getting the water they need, or are they getting something else with the water?


 Sound can change according to the speed at which it travels. Another word for sound speed is pitch. When the sound speed slows, the pitch lowers. With clarinet reeds, it’s high. Guitar strings can do both, as they are adjustable. If you look carefully, you can actually see the low pitch strings vibrate back and forth, but the high pitch strings move so quickly it’s hard to see. But you can detect the effects of both with your ears.
Sound can change according to the speed at which it travels. Another word for sound speed is pitch. When the sound speed slows, the pitch lowers. With clarinet reeds, it’s high. Guitar strings can do both, as they are adjustable. If you look carefully, you can actually see the low pitch strings vibrate back and forth, but the high pitch strings move so quickly it’s hard to see. But you can detect the effects of both with your ears. So why do we hear a boom at all? Sonic booms are created by air pressure (think of how the water collects at the bow of a boat as it travels through the water). The vehicle pushes air molecules aside in such a way they are compressed to the point where shock waves are formed. These shock waves form two cones, at the nose and tail of the plane. The shock waves move outward and rearward in all directions and usually extend to the ground.
So why do we hear a boom at all? Sonic booms are created by air pressure (think of how the water collects at the bow of a boat as it travels through the water). The vehicle pushes air molecules aside in such a way they are compressed to the point where shock waves are formed. These shock waves form two cones, at the nose and tail of the plane. The shock waves move outward and rearward in all directions and usually extend to the ground.
 Sound is everywhere. It can travel through solids, liquids, and gases, but it does so at different speeds. It can rustle through trees at 770 MPH (miles per hour), echo through the ocean at 3,270 MPH, and resonate through solid rock at 8,600 MPH.
Sound is everywhere. It can travel through solids, liquids, and gases, but it does so at different speeds. It can rustle through trees at 770 MPH (miles per hour), echo through the ocean at 3,270 MPH, and resonate through solid rock at 8,600 MPH.

 This is the experiment that all kids know about… if you haven’t done this one already, put it on your list of fun things to do. (See the tips & tricks at the bottom for further ideas!)
This is the experiment that all kids know about… if you haven’t done this one already, put it on your list of fun things to do. (See the tips & tricks at the bottom for further ideas!)

 What you’ve done is create a transverse wave. With a transverse wave, if the particle (in this case your hand) moves up and down, the wave will move to the left and/or right of the particle. The word perpendicular means that if one thing is up and down, the other thing is left and right. A transverse wave is a wave where the particle moves perpendicular to the medium. The medium is the material that’s in the wave. The medium in this case is the rope.
What you’ve done is create a transverse wave. With a transverse wave, if the particle (in this case your hand) moves up and down, the wave will move to the left and/or right of the particle. The word perpendicular means that if one thing is up and down, the other thing is left and right. A transverse wave is a wave where the particle moves perpendicular to the medium. The medium is the material that’s in the wave. The medium in this case is the rope. Here you made a longitudinal wave. A longitudinal wave is where the particle moves parallel to the medium. In other words, your hand vibrated in the same direction (parallel to the direction) the wave was moving in. Your vibrating hand created a wave that was moving in the same direction as the hand was moving in. Did you take a look at the tape? The tape was moving back and forth in the same direction the wave was going.
Here you made a longitudinal wave. A longitudinal wave is where the particle moves parallel to the medium. In other words, your hand vibrated in the same direction (parallel to the direction) the wave was moving in. Your vibrating hand created a wave that was moving in the same direction as the hand was moving in. Did you take a look at the tape? The tape was moving back and forth in the same direction the wave was going.
















 What’s going on? We’re utilizing the “springy-ness” in the popsicle stick to fling the ball around the room. By moving the fulcrum as far from the ball launch pad as possible (on the catapult), you get a greater distance to press down and release the projectile. (The fulcrum is the spot where a lever moves one way or the other – for example, the horizontal bar on which a seesaw “sees” and “saws”.)
What’s going on? We’re utilizing the “springy-ness” in the popsicle stick to fling the ball around the room. By moving the fulcrum as far from the ball launch pad as possible (on the catapult), you get a greater distance to press down and release the projectile. (The fulcrum is the spot where a lever moves one way or the other – for example, the horizontal bar on which a seesaw “sees” and “saws”.)



