Cool Blue Light Chemiluminescence Experiment
Glow sticks generate light with very little heat, just like the glow you see from fireflies, jellyfish, and a few species of fungi. Chemiluminescence means light that comes from a chemical reaction. When this happens in animals and plants, it’s called bioluminescence. In a glow stick, when you bend it to activate it, you’re breaking … Continue reading "Cool Blue Light Chemiluminescence Experiment" |
Special Science Teleclass: Chemistry & Chemical Engineering
This is a recording of a recent live teleclass I did with thousands of kids from all over the world. I’ve included it here so you can participate and learn, too! We’re going to be mixing up dinosaur toothpaste, doing experiments with catalysts, discovering the 5 states of matter, and building your own chemistry lab … Continue reading "Special Science Teleclass: Chemistry & Chemical Engineering" |
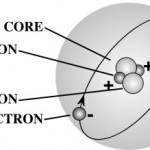
Atomic Facts
A gram of water (about a thimble of water) contains 1023 atoms. (That's a '1' with 23 zeros after it.) That means there are 1,000,000,000,000,000,000,000,000 atoms in a thimble of water! |
Bubble Experiments
If you’re fascinated by the simple complexity of the standard soap bubble, then this is the lab for you. You can easily transform these ideas into a block-party Bubble Festival, or just have extra fun in the nightly bathtub. Either way, your kids will not only learn about the science of water, molecules, and surface … Continue reading "Bubble Experiments" |
Cobalt Colors
Cobalt chloride (CoCl2) has a dramatic color change when combined with water, making it a great water indicator. A concentrated solution of cobalt chloride is red at room temperature, blue when heated, and pale-to-clear when frozen. The cobalt chloride we’re using is actually cobalt chloride hexahydrate, which means that each CoCl2 molecule also has six … Continue reading "Cobalt Colors" |
Cool Milk Trick
Have you ever tried washing dishes without soap? It doesn’t work well, especially if there’s a lot of grease, fat, or oil on the dish! The oils and fats are slippery and repel water, which makes them a great choice for lubration of bearing and wheels, but lousy for cleaning up after dinner. So what’s … Continue reading "Cool Milk Trick" |
Desalination
This experiment is for advanced students. Lewis and Clark did this same experiment when they reached the Oregon coast in 1805. Men from the expedition traveled fifteen miles south of the fort they had built at the mouth of the Columbia River to where Seaside, Oregon now thrives. In 1805, however, it was just men … Continue reading "Desalination" |
Dinosaur Toothpaste
Hydrogen peroxide is used to fuel rockets, airplanes, and other vehicle engines. Chemistry teachers everywhere use it to demonstrate the power of a catalyst. To speed up a reaction without altering the chemistry of the reaction involves adding a catalyst. A catalyst changes the rate of reaction but doesn’t get involved in the overall chemical … Continue reading "Dinosaur Toothpaste" |
Electroplating
If you don’t have equipment lying around for this experiment, wait until you complete Unit 10 (Electricity) and then come back to complete this experiment. It’s definitely worth it! Electroplating was first figured out by Michael Faraday. The copper dissolves and shoots over to the key and gets stuck as a thin layer onto the … Continue reading "Electroplating" |
Disappearing Foam Cup
This is looks like a chemical reaction but it's not - it's really just a physical change. It's a really neat trick you can do for your friends or in a magic show. Here's how it works:Supplies:acetonepie pan (or other shallow baking dish)Styrofoam cup |
Football Ice Cream
Is it hot where you live in the summer? What if I gave you a recipe for making ice cream that doesn’t require an expensive ice cream maker, hours of churning, and can be made to any flavor you can dream up? (Even dairy-free if needed?) If you’ve got a backyard full of busy kids … Continue reading "Football Ice Cream" |
Magnesium Battery
Magnesium is one of the most common elements in the Earth’s crust. This alkaline earth metal is silvery white, and soft. As you perform this lab, think about why magnesium is used in emergency flares and fireworks. Farmers use it in fertilizers, pharmacists use it in laxatives and antacids, and engineers mix it with aluminum … Continue reading "Magnesium Battery" |
PVA Slime
Instead of using glue as a polymer (as in the slime recipes above), we're going to use PVA (polyvinyl alcohol). Most liquids are unconnected molecules bouncing around. Monomers (single molecules) flow very easily and don't clump together. When you link up monomers into longer segments, you form polymers (long chains of molecules).Polymers don't flow very … Continue reading "PVA Slime" |
Rusty Balloon
Mars is coated with iron oxide, which not only covers the surface but is also present in the rocks made by the volcanoes on Mars. Today you get to perform a chemistry experiment that investigates the different kinds of rust and shows that given the right conditions, anything containing iron will eventually break down and … Continue reading "Rusty Balloon" |
Taking the Salt out of the Ocean
This experiment is for advanced students.Have you ever taken a gulp of the ocean? Seawater can be extremely salty! There are large quantities of salt dissolved into the water as it rolled across the land and into the sea. Drinking ocean water will actually make you thirstier (think of eating a lot of pretzels). So … Continue reading "Taking the Salt out of the Ocean" |
Turning Water into Ink
You can use this as real ink by using it BEFORE you combine them together like this: dip a toothpick into the first solution (sodium ferrocyanide solution) and with the tip write onto a sheet of paper. While the writing is drying, dip a piece of paper towel int other solution (ferric ammonium sulfate solution) … Continue reading "Turning Water into Ink" |
Turning Water into Wine
Phenolphthalein is a weak, colorless acid that changes color when it touches acidic (turns orange) or basic (turns pink/fuchsia) substances. People used to take it as a laxative (not recommended today, as ingesting high amounts may cause cancer). Use gloves when handling this chemical, as your skin can absorb it on contact. I’ll show you … Continue reading "Turning Water into Wine" |
Water Purification
Ever wonder how the water draining down your sink gets clean again? Think about it: The water you use to clean your dishes is the same water that runs through the toilet. There is only one water pipe to the house, and that source provides water for the dishwasher, tub, sink, washing machine, toilet, fish … Continue reading "Water Purification" |
Working with Cataylsts
This experiment is for advanced students. Don’t put this in your car….yet. Hydrogen generation, capture, and combustion are big deals right now. The next phase of transportation, and a move away from fossil fuels in not found in electric cars. Electric cars are waiting until hydrogen fuel cell vehicles become practical. It can be done … Continue reading "Working with Cataylsts" |
Acids and Bases
This experiment is for advanced students. ACID!!! The word causes fear to creep in and get our attention. BASIC!!! The word causes nothing to stir in most of us. The truth is, a strong acid (pH 0-1) is dangerous, but a strong basic (pH 13-14) is just as dangerous. In this lab, we will get … Continue reading "Acids and Bases" |
Carbon Dioxide
This experiment is for advanced students. This lab builds on concepts from the previous carbon dioxide lab. Limewater….carbon dioxide…indicators. We don’t know too much about these things. Sure, we know a little. Carbon dioxide is exhaled by us and plants need it to grow. Burning fossil fuels produces carbon dioxide. Indicators…something we observe that confirms … Continue reading "Carbon Dioxide" |