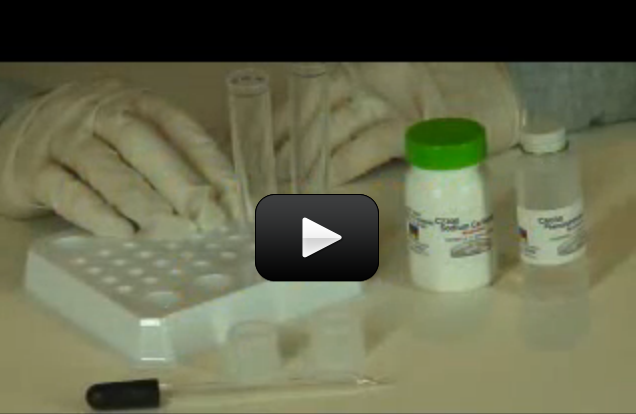
Phenolphthalein is a weak, colorless acid that changes color when it touches acidic (turns orange) or basic (turns pink/fuchsia) substances. People used to take it as a laxative (not recommended today, as ingesting high amounts may cause cancer). Use gloves when handling this chemical, as your skin can absorb it on contact. I’ll show you how:
Please login or register to read the rest of this content.

Hi there! So sorry for the confusion!
Phenolphthalein is an indicator of acids and bases. It’s colorless with acids and turns pink with bases. Phenolphthalein is used to test in the range of pH of about 8-10. I apologize for an error s- I’ll check on that and get it corrected right away.
Hi there, we had a blast with this experiment. Along with the sodium carbonate solution, we tried: imitation vanilla flavor, soy sauce, Frank’s Red Hot, lemonade, sodium bicarbonate (baking soda) solution, and soapy water. We found that some were acids, some were bases. We did, however, have a little confusion as to how we would know if our liquids were an acid or a base since in the student worksheet you said,” You should understand that phenolphthalein is an acid/base indicator that stays clear in the presence of an acid but turns colors in the presence of a base.” but on the experiment page with the video on it you said,”Phenolphthalein is a weak, colorless acid that changes color when it touches acidic (turns orange) or basic (turns pink/fuchsia) substances.” Does it stay clear when it touches an acid or does it turn orange? Based on the rest of the information, we concluded that phenolphthalein turns clear when it touches an acid and changes color when in contact with a base.
Thanks Aurora,
The Short kids
ok thanks aurora 🙂
NO!!!! Don’t ever drink ANYTHING in ANY science experiment ever!! Big no-no! 🙂
Good to ask!
can you drink this aurora ?
cool I’m gonna like this
I used cabbage juice instead of phenolphthalien and a lemon juice/water mix rather than the washing soda. It turned out great! I also tried it with baking soda and water and it turned blue.
No, it’s a solution of chemicals. DO NOT DRINK!
Is this real wine?
You can find it here. Please make sure to handle this with gloves on!
Where do you get Phenolphthalein?
I’ll have my team contact you right away!
Where is that science experiment manual you mention?
I’m not sure if this will answer your questions or not, but…..blue litmus paper turns red under acidic conditions and red litmus paper turns blue under basic conditions. Phph in a base turns pink at first, but will turn purple as the conditions become more basic…..as I understand it, anyway.
Blue litmus paper turns red under acidic conditions and red litmus paper turns blue under basic
I think we found the answer to the question at the website below: http://www.elmhurst.edu/~chm/vchembook/186indicator.html
Thanks.
Thanks for the response. Now I have a new question. We have learned that acids turn pink/red and bases turn blue/purple when tested with a Ph Indicator. However, Phph turning pink with a base solution has us confused. It seems that the color depends on the testing agent. Therefore, a litmus paper will turn blue with a base but Phph will turn pink with the same base. Is that right?
Phenolphthalein is prepared by dissolving Phenolphthalein powder in alcohol, making the solution acidic. Therefore, phenolphthalein (phph) is colorless in the presence of an acid. In a basic solution, phph turns pink, and in more basic solutions, turns purple. Tumeric solution stays yellow in the presence of an acid and changes purple-brown in the presence of a base.
It has to do with hydrogen ions. An acid is a substance that donates hydrogen ions. A base is looking to absorb, to gain, hydrogen ions. In the case of phph in an acid, both solutions are hydrogen ion donators and there is no color change. With turmeric and an acid, again there is no color change for the same reason. There is a color, but that is just the usual yellow color of turmeric solution. For more information, look up Le Chatelier’s Principle.
The experiment worked when performed as shown on the video. However, when we added some phenolpthalein to Vinegar (acetic acid), it did not change color – nothing happened. Using Turmeric as indicator worked but not phenolpthalein. Does it have some limitations?
i’m gonna love this